The Antibody-Drug Conjugate Challenge
The idea of arming antibodies with other molecular moieties such as toxins or chemotherapeutics dates back to the 1980s, when such antibodies were first being developed for clinical use. Today, so called ”naked” antibodies are being harnessed to fight cancer cells in many different forms – several of which have become blockbuster therapies. Theoretically, if coupled to cytotoxic substances, antibodies could become even more powerful while eliciting fewer side effects. Despite these hypothetical advantages and the outlay of huge R&D efforts, particularly in oncology, few antibody-drug conjugates (ADCs) exist in today’s market. In 2000, gemtuzumab ozogamicin – intended to treat acute myeloid leukemia – became the first ADC to make it to the clinic. It was voluntarily withdrawn from the market in 2012 after post-marketing surveillance data showed it did not improve the chances of survival, but heightened the risk of fatal toxicity.
BRENTUXIMAB VEDOTIN
In February 2011, brentuximab vedotin of Seattle Genetics was approved by the FDA. It is the only ADC currently on the market. This antibody is directed against membrane protein CD30, present on the white blood cells of Hodgkin’s lymphoma. Linked to the antibody are three to five molecules of the toxin monomethyl auristatine E (MMAE, vedotin). This powerful tubulin-inhibitor strongly impairs cell division and ultimately leads to apoptotic cell death. Vedotin is so toxic to healthy tissues that it cannot be used as a stand-alone chemotherapeutic.
In this preparation, it is coupled to brentuximab by a bridge of two amino acids. When brentuximab vedotin binds to CD30, the ADC-CD30 complex is internalized and transported to the lysosomes. Proteases within the lysosomes split the amino acid bridge and the deadly payload becomes active at its place of destination: in the CD30-positive white blood cell.
THE LATEST GENERATION ADCs
Three building blocks – (1) antibody, (2) linker and (3) toxin – are characteristic of all ADCs. Dozens of ADCs are currently undergoing clinical trials for the treatment of a variety of cancers. Many others are making their way through preclinical development.
This increased attention to ADCs has been triggered by advances in linker chemistries. Modern linker chemistries with enhanced stability have been developed to ensure that ADCs remain intact until they reach their target cells. The dissociation of modern linker chemistries is driven by enzymatic degradation in the target cell, whereas early generation dissociation was a pH-dependent process. For instance, MylotargTM‘s hydrazon linker was stable at neutral blood pH, but would dissociate at the lower pH levels found in lysosomes. Despite this fail-safe, a fair percentage of the toxin was liberated in the blood stream. New linker chemistries are about ten times more stable than previous generations.
THE FUTURE FOR ADCs
These recent advancements in linker chemistries have led a lot of biotech companies to take another look at their portfolios of “naked” antibodies. Antibodies that have failed in Phase I or II are being scrutinized again. In addition to novel antibodies, already approved antibodies are also being used as bases for ADCs. After positive Phase II studies, Genentech is now conducting Phase III studies for an ADC of trastuzumab linked to the cytotoxic agent, emtansine in patients with HER2-positive breast cancer. The company has recently filed a Biologics License Application (BLA) with the US FDA.
The advantage of using a therapeutically proven antibody agent such as trastuzumab is that there is already a lot known about the antibody itself as well as its pharmacological target, which in this case is the tumor cell membrane protein HER2.
The efficacy of an ADC is largely dependent upon how many copies of the target are expressed on the tumor cells and how fast the ADC is being transported into the tumor cell. In general, rather high copy numbers (> 100,000 per cell) are required to import enough toxin to kill the cell. Despite advances in ADC engineering, only a fraction of the total injected antibody dose can be effectively delivered to the target tumor. Hence the cytotoxic agent bound to the antibody needs to be highly potent so that any ADCs that do reach their target cells have the maximum killing potential.
This highly complex balancing act is typical for the development of ADCs, which not only requires the merging of biotechnology and organic chemistry derived technologies but also requires a thorough understanding of the biological complexity of the targets as well as the handling of extremely potent toxins. Nonetheless, future prospects for ADCs seem to be bright even though it has been hard to predict how certain tumor types will actually respond to treatment. Only clinical trials can predict how well this promise will pay off to benefit patients.
THE COMPETITIVE LANDSCAPE FOR ADCs
Convinced that ADCs have extensive potential for therapeutic applications and may present significant medical breakthroughs over the coming decade, particularly in cancer treatment, several companies, both big and small, are active in the field.
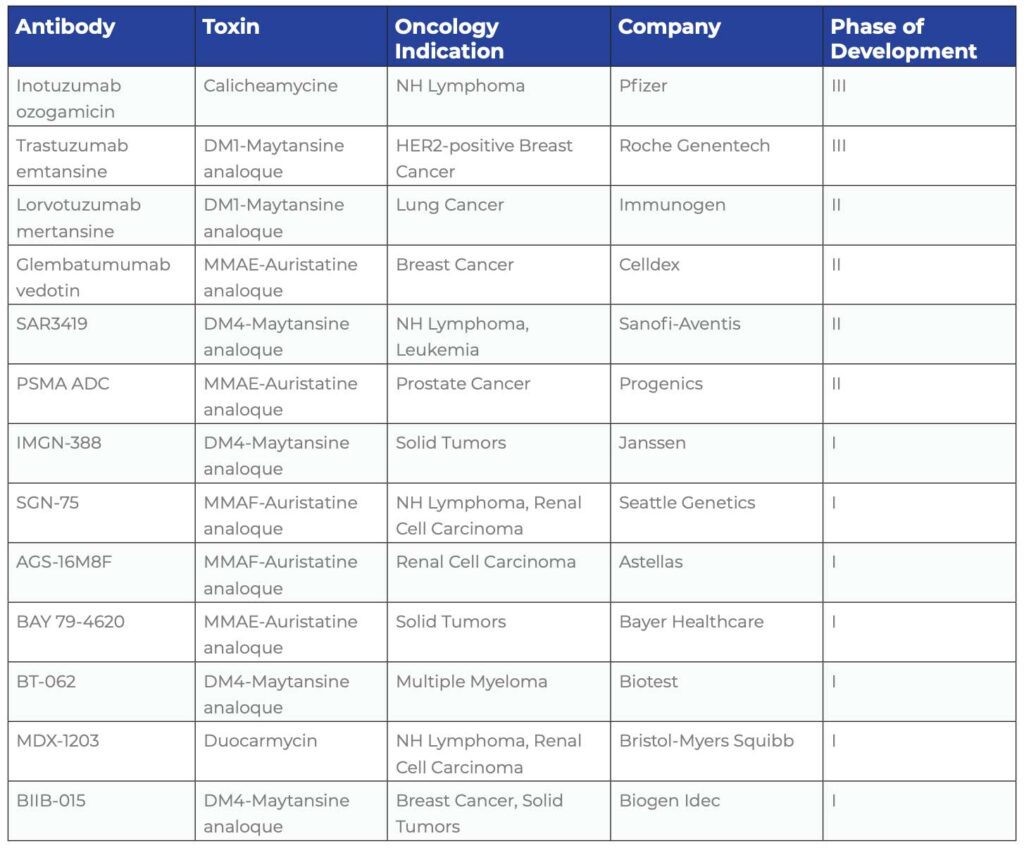
CURRENT PHARMACEUTICAL COMPANY ACTIVITY IN ADC DEVELOPMENT
There is no longer any doubt that ADC technology works, and many antibodies have been developed to bind to a wide variety of antigens. These new antibodies only need additional factors to turn them into effective and potent therapies. The next few years will likely bring impressive advances in the various pieces of ADC technology: the antibodies, the drugs, the linkers that bind them together, and newfound targets for the ADCs to attack.
The two technology platform pioneers in the field, Seattle Genetics and ImmunoGen, have many collaborators. Seattle Genetics is working with Genentech, Millennium, GlaxoSmithKline, Celldex, Progenics, Bayer, Daiichi Sankyo, MedImmune and Astellas. ImmunoGen is working with Genentech, Sanofi-Aventis, Amgen, Biogen Idec, Biotest and Bayer.
QPS IS COMMITTED TO WORKING WITH YOU
QPS has extensive experience in supporting ADC drug development. We understand the complexities, particularly with respect to managing and conducting global clinical oncology, proper bioanalysis, and monitoring the pharmacokinetics of ADC drug candidates. We are committed to working with you personally to advance your ADC product for the benefit of patients worldwide.
BROAD ACCESS
QPS provides clients with broad access to our nonclinical and clinical development capabilities. Clients also benefit from our experience in nonclinical and clinical development of a diverse portfolio of treatment modalities for a wide range of oncology and other indications. Our preferred vendor agreements also provide for the establishment of client-dedicated units within our organization.
TIMELY DELIVERY
Partnering with QPS will position your company for success, enabling timely, personalized delivery of your ADC portfolio to the marketplace.








